SYNTHGENE SARS-COV-2 ANTIGEN RAPID TEST
Introduction
SYNTHGENE SARS-CoV-2 Nucleocapsid(N) Antigen Rapid Detection Kit (Colloidal gold method) is registered and approved for use in Singapore by the Health Science Authority (HSA) w.e.f 13 Sep 2023. The test does not require skilled professional or equipment to interpret the test results and is easy and simple to use, suitable for both home and office use.
SYNTHGENE SARS-CoV-2 Nucleocapsid(N) Antigen Rapid Detection Kit (Colloidal gold method) uses colloidal gold immunochromatography combined with the double-antibody sandwich principle to detect the N-antigen of novel coronavirus in human nasal and nasopharyngeal swab specimens.
- Nasal and Nasopharyngeal swab specimens
- Results in 10-15 minutes
- High Accuracy
- Easy to use
- No equipment needed
- Room temperature storage
Our Strategic Partner
Our strategic partner; Nanjing Synthgene Medical Technology Co., Ltd., is a biomedical high-tech enterprise focusing on the development and production of OTC home self-inspection products. The company has a mature technology platform, including chromatography platform, isothermal amplification platform, etc. The product line covers infectious disease detection, pregnancy diagnosis, drug detection, pet disease diagnosis, gastrointestinal function detection and other fields. At the same time, the company is also developing smart detection products suitable for home self- testing.
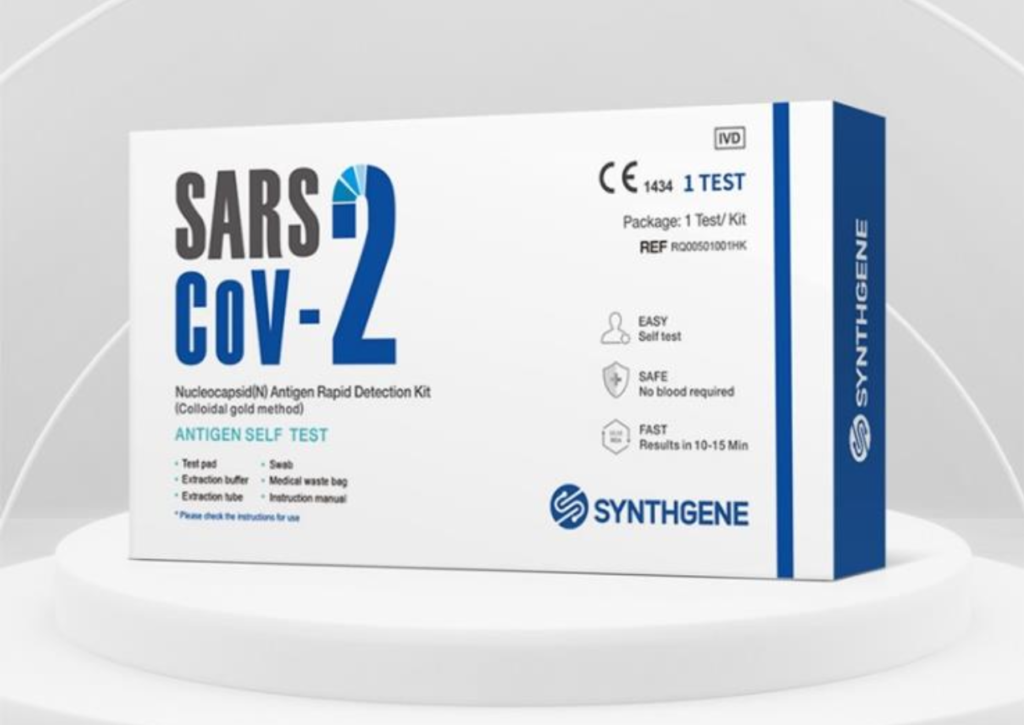
Swab Procedure

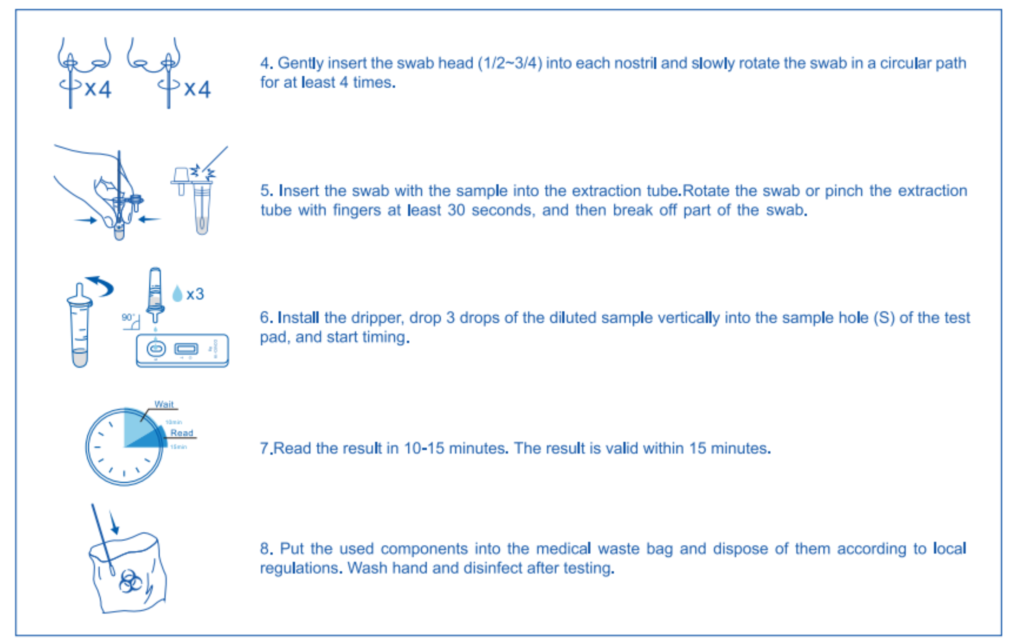
Results Interpretation